
Around 209 BCE, China’s emperor, Qin Shi Huang, ordered the construction of an enormous army with over 8,000 warriors, chariots, archers, strongmen, and acrobats. However this army did not consist a single man or woman, rather it was built purely of stone and time.
This wasn’t a force aimed at any specific country or army. Rather it was Qin’s army to defend himself against death, and personally accompany him into the afterlife; Emperor Qin wanted to live forever.
This was a man who commanded so much power, that he singlehandedly ordered the construction of the Great Wall that we all know today. Nevertheless emperor Qin died at age 49, no closer to immortality than any of his servants or soldiers.
One Day, We Will All Die.
Seems simple enough right?
We’re thinking about a universal truth that everybody knows and is going to experience. It seems unthinkable to question why we die, or how we can outsmart our own end. But yet throughout history humans have continued to ponder about death, and many have even dreamed of escaping death altogether by divine means.
Throughout most of time, the summary of your life would start at your birth, mature with growth and mating, wind down with the raising of your children, and end with your death. We view death and aging as something to never question, yet it’s something that we as people can’t stop questioning nonetheless.
The process of aging would take you from the beginning of your life to its end like a soulless conveyor belt that you, nor anyone around you could hop off of.
Is Aging Really Inevitable?…
The reason we have been conditioned to think of death as inevitable and natural, is really because there was never an alternative to it; we know it as a fundamental truth.
However many people are starting to look at the entire premise of aging and death a little differently.As the key mechanisms that dictate aging as we know it are unraveled, we‘re beginning to think of ways to get around them. The significant amounts of money and people who are jumping on solving aging just shows the very real effort that exists to extend human longevity.
This all points to how we’re beginning to look at aging not as the inevitable end, but rather now as a disease like cancer or ALS.

Laura Deming is one of SIlicon Valley’s youngest venture capitalists, who is well known for her funding of numerous anti aging projects.
Extending the Human Healthspan
When it comes to what the goal of extending human longevity really is, you may imagine trying to get people living to age 180 in some sci-fi world. However if you think about it, it’s not in anyone’s best interest to live up to an insane age, if they’re still going to face the worsening brunt of aging such as dementia, brittleness, etc.
This is why new Longevity solutions often focus on expanding the human healthspan. The term “healthspan” means the ability of a person to maintain a longer-lasting time frame of strong health as they age. This idea is being viewed more and more as the viable alternative to simply “prolonging life”, and it focuses more on mitigating aging’s effects.
After all, what’s the point of living 180 years, only to have many of your memories and senses long gone by age 90?
Inevitably by pushing the bar of the human healthspan, we are also prolonging the years people will live by small increments. However the main concern is focused on mitigating the effects of aging, for a higher quality of life; making 90 years old the new 60.
The Science of Aging
Aging as a mechanism is incredibly complex, mainly because your entire body is involved with its progression. Although the mechanisms of aging encompass so many different domains of study and research, scientists often point everything back to one main culprit: telomere shortening.
Telomere Shortening
Inside every cell in the body, our DNA is scaffolded into long and elaborate structures known as chromosomes. Chromosomes hold most of the genetic information that our cells work with, but have one key feature that we’re really interested in when it comes to aging.
Let’s imagine our chromosomes as the shoelaces on your favorite pair of sneakers.
Unlike the shoelaces though, your telomeres decay over time!
Just like how your shoelaces have those mini plastic caps on their edges, your chromosomes have DNA caps on their own structural edges. These chromosome caps are what we call Telomeres, and they’re a lot more important to you than any shoelaces.
Telomeres are large areas of non-coding, repeating sections of DNA on the edges of the chromosome. Now although these areas don’t code for anything, they are far from useless. As a matter of fact, these telomeres are crucial for keeping you alive and well!
The Haflick Limit
From the moment you are born, the chromosmes in your fresh cells are beaming with long, healthy telomeres. However every time your cells divide from this point on, your telomeres decay bit-by-bit. So as time elapses as your cell undergoes more divisions, your telomeres gradually shrink.
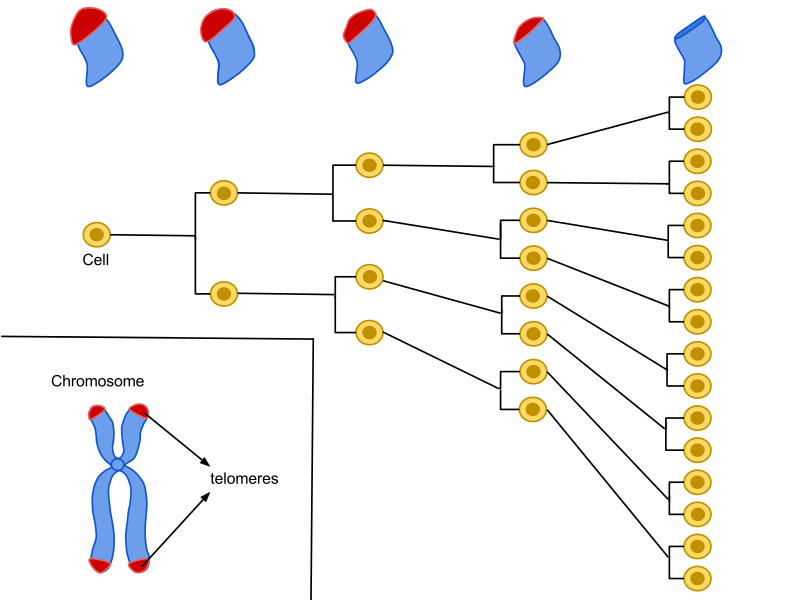
Here you can see the Hayflick limit being reached at the final stage illustrated (Stage 4)
Eventually your cell has an existential crisis, as it cannot carelessly divide anymore with little-to-no telomeres left. This cell division limit is what we call a Hayflick limit.
Once your cells hit their Hayflick limit, they get stuck in an existential crisis, where they need to decide between two fates:
- Keep Dividing: In this case, the cell will keep dividing until its telomeres are completely decayed. With no essential “plastic cap”, our shoelace of chromosome begins losing its own coding DNA every time the cell divides.
- Cellular Senescence: Here our cell decides to obey the law, and follow its Hayflick Limit. From this point on, our cell will enter a “zombie state” known as a senescent cell, where it will not die on its own, nor’ will it do anything.
If the cell chooses to keep dividing, it puts itself at risk of dying early, or worse, even becoming cancerous. However as a protection mechanism for the greater good of your body, our cells usually choose cellular senescence as their fate. This prevents them from becoming rogue and cancerous, but it also puts the cell into a useless zombie state, where the cell will not die.
Knowing that aging is primarily caused by our telomeres wearing down after every cell division, the question arises as to how we can reverse this process.
Telomerase and Cellular Immortality
If the degradation of the telomeres is what causes aging in all our cells, it’s intuitive that by rebuilding the telomeres, you could create an immortal cell! It just so happens that we‘re familiar with stem cells and cancer cells: two familiar types of immortal cells that don’t lose their telomeres as they divide.
What makes these cells special from all the other cells is an enzyme called telomerase, which is being credited as the primary reason why immortal cells can cheat aging and death as we know it. Telomerase is responsible for rebuilding the portion of telomeres that decay after each cell division, by replacing missing DNA base pairs to the telomere DNA sequence that was lost.
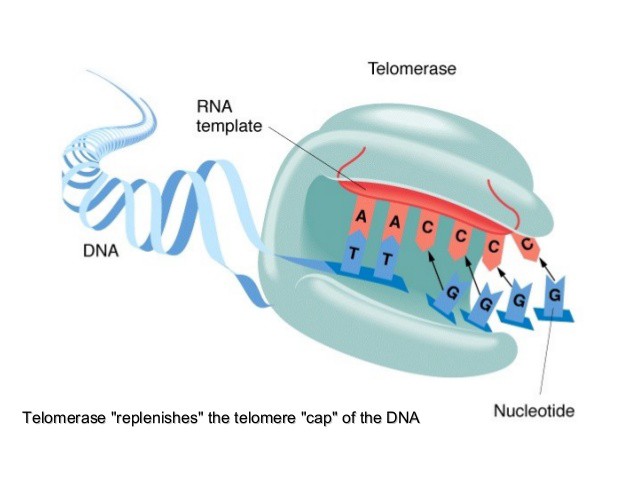
PC: Kashmeera N.A. @SlideShare
By having this enzyme consistently rebuilding the cell’s telomeres as they decay, we’re essentially removing the Hayflick limit from our cell, thus granting it a new found freedom of immortality. Knowing this, my team and I set out to find a way to express telomerase in somatic cells, which consist of the majority of cells in your body from your skin to your stomach.
These mortal cells repair and replace one another in the forms of entire tissues, and are the building blocks for most parts of the body.
Expressing Telomerase In Somatic Cells
Our team set out to find a new way of trying to create immortal somatic cells, by the means of causing telomerase to be produced within them. Our means of activating telomerase production would be done by manipulating the expression of an important gene which indirectly codes for telomerase, known as “Human telomerase reverse transcriptase”, or hTERT.
You can check out our research proposal here.
The hTERT Gene
In human stem cells with cellular immortality, the hTERT gene is an active gene which codes for a key part of telomerase, allowing it to proliferate in the volumes needed to restore the chromosome telomeres after every division. The main problem from here is that somatic cells have their of the hTERT gene repressed, meaning the gene cannot code for telomerase like we need.
But why does hTERT have a hard time in somatic cells, but not in stem cells?
This all comes down epigenetics, which is essentialy how our body interacts and manipulates the genome, without actually changing any DNA base pair sequences. Specifically we are looking at special tags placed on a coiled DNA structures, known as histones.
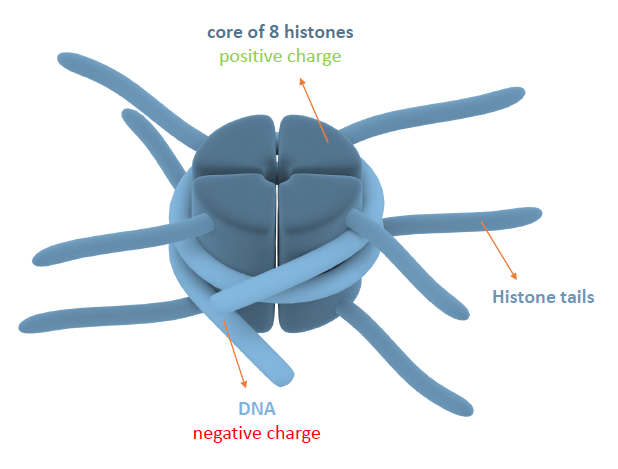
DNA wraps around histones like a hose around a spool.
In somatic cells, the histone that hTERT belongs to has a couple of added chemical tags, known as histone methylation marks. These chemical marks lower or completely stop the activity of whichever genes are tied in the histone, including the hTERT gene we are trying to keep active.
Our Idea: Stop Whatever Maintains The Inactive Marks
My team and I figured that if we stopped the maintenance of one of these repressive histone marks, the hTERT gene would be more actively expressed. In order to go about this, we needed to identify which specific enzyme is responsible for placing down the repressive histone mark we chose, as well as an inhibitor molecule for that enzyme.
The end goal would higher expression of the hTERT gene, which would then allow for telomerase to be produced in our somatic cell, giving it cellular immortality.
A key thing to note here is that although we would eliminate the Hayflick Limit, immortal somatic cells would not turn cancerous, as proven in mice models. Cells would require high numbers of genetic insults, on top of replicative immortality, to become cancerous.
Here was our ideation process in a nutshell:
- After identifying a couple of these inactive histone marks, we singled down a specific histone mark known as H3K9Me3. This specific mark is key in indirectly repressing how active hTERT is as a gene.
- Afterwards we identified two enzymes that lay down and maintain H3K9Me3 near the telomeres, called SUV39H1 and SUV39H2.
- Finally we found a specific molecule which can inhibit SUV39H1, and very likely SUV39H2 as well, known as Chaetocin.
The main concern with our Chaetocin molecule is that it’s an inhibitor which is not incredibly specific for just targeting SUV39H1. We figured we could leverage this unspecific nature of Chaetocin to target the enzyme SUV39H2 as well, which differs very little from SUV39H1. However to prevent serious side effects, our team concluded that we would need a highly accurate drug delivery mechanism for this treatment.
Essentially, we figured that if we accurately block the enzymes SUV39H1/H2 from placing the inactive H3K9Me3 mark on hTERT’shistone, we should get more hTERT expression. The more hTERT gene expression means more telomerase, and more telomerase expression means a higher chance of getting an immortal cell!
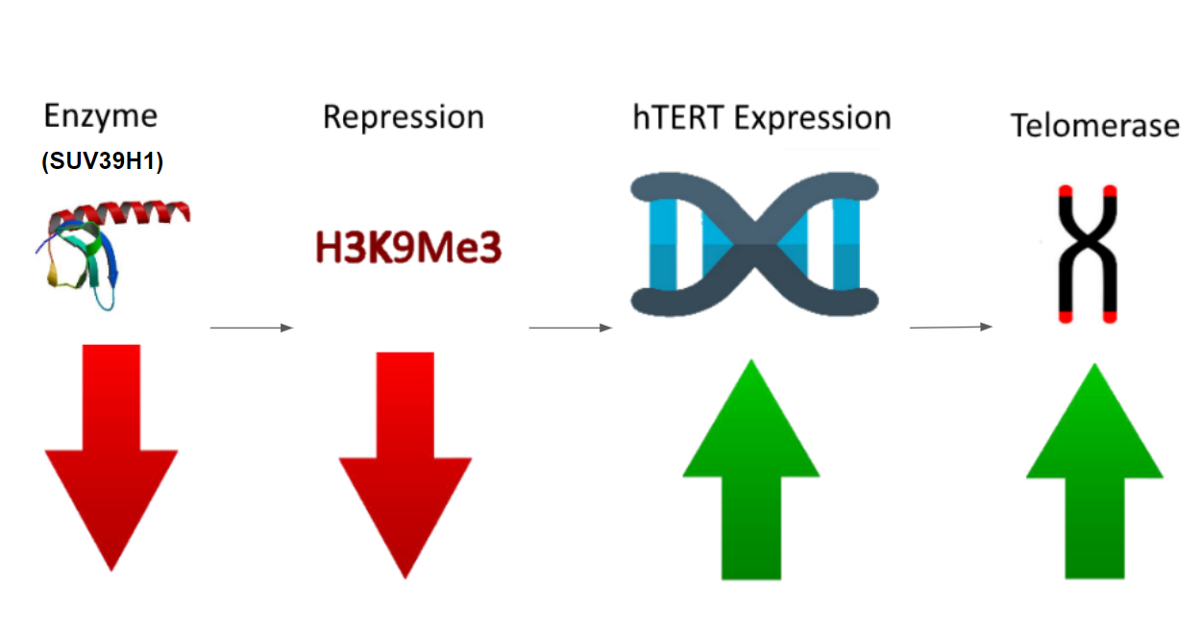
The Logic Model of our Experiment Idea
The Final Product
Finally after we had our completed logic model and test drug, we had to address the elephant in the room: How are we going to be able to deliver Chaetocin to its target enzymes?
A huge concern when it comes to delivering any drug inside the human body, is the potential for drug side effects, as well as immune responses. If your body and cells detect the drug as a potential invading threat, doesn’t matter what the real intention of the drug is, your body is going to try to destroy the invader.
In the context of how your body reacts to drugs, your health is at risk of everything from absolutely nothing, to a deadly immune response.
This is where we decided to implement a well known drug delivery tool called Liposomes. These are essentially lipid bubbles with an empty molecular core, allowing for drug molecules to be placed inside. The outside lipid layer allows the drug to be delivered safely to the target, and the empty core inside holds the drug molecule until delivery.
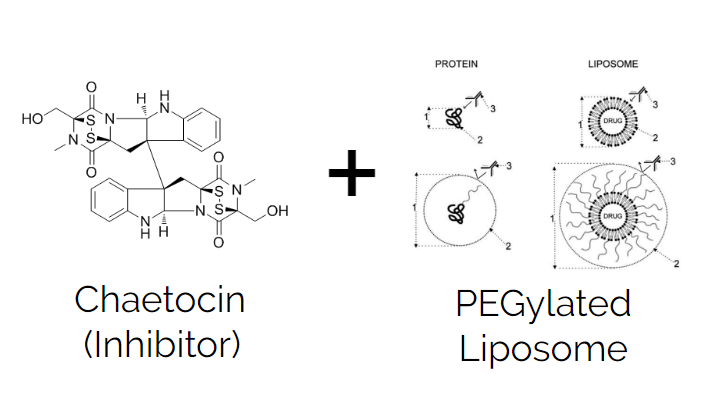
The Dynamic Duo of our Model: Use a PEGylated Liposome with it’s hollow core, to hold the Chaetocin molecule inside.
In order to further boost the liposome’s drug delivery abilities, we propose coating them in a layer of a chemical known as Polyethylene Glycol (aka Margol), turning our regular Liposome into a “PEGylated Liposome”.
These upgraded liposomes last longer in the body, as well as have a drastically reduced chance of causing immune responses in the patient’s body! Areas that still need to be figured out are: navigation inside the nucleus, as well as the timing as to when the liposome should release the Chaetocin payload.
Key Takeaways and Final Thoughts
As we understand aging more and more as a biological function, rather than some sort of inevitable philosophical fate, we begin to look at it for what it really is: the slow degradation and wasting away of our physical being. By creating this idea and experimental model, we hope to contribute to this evergrowing fight, to extend the human healthspan.
- The primary cause of aging in humans is telomere shortening, which occurs in almost all of our cells except for stem cells and cancer cells, which are immortal.
- Telomerase is an enzyme that rebuilds telomeres everytime they decay, essentialy giving any cell that produces telomerase, immmortality.
- Telomerase’s main gene, hTERT, is epigenetically silent in mortal cells, while active in immortal cells. By removing a chemical mark which represses hTERT in somatic cells, we predict we can help kickstart telomerase production in them!
- The end optimal result would be to get a somatic cell with cellular immortality, as it would not have hTERT repressed, and would be able to produce sufficient telomerase to consistently repair its own telomeres.
It’s a lot harder to fathom a world without death, than a world with it; the process of aging and death is something we are conditioned biologically to live by and accept.
Pushing the bar of the human healthspan further and further is the way we actually approach this anti aging dream that most of us have thought about at some point in our life. We will almost certainly all live to see the day where anybody with enough money and the intention, be allowed the privilege to survive far beyond their current biological limits.
In the coming years, the more this sentiment of viewing aging as a disease is pushed, the closer we will be to making 90 years old, the new 60. Humanity will eventually fulfill its curiosity in immortality, and we’ll look at death as intrigued as when we look at Emperor Qin’s afterlife army today.
All Rights Reserved for Michael Trinh
